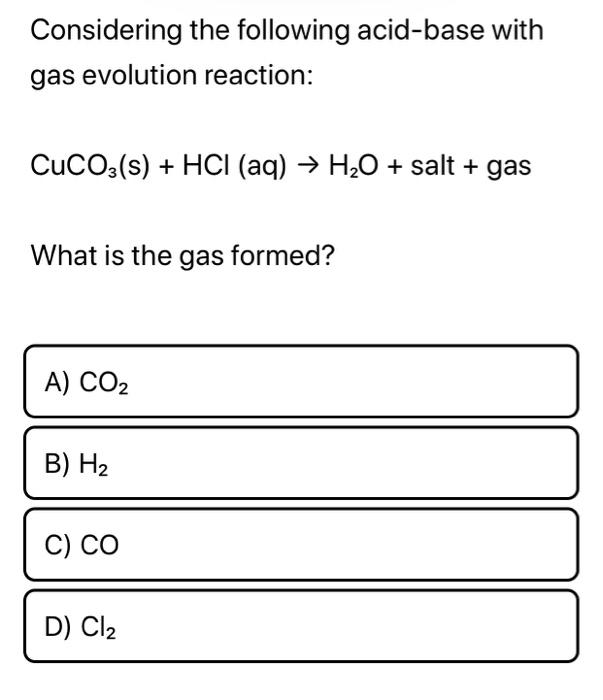
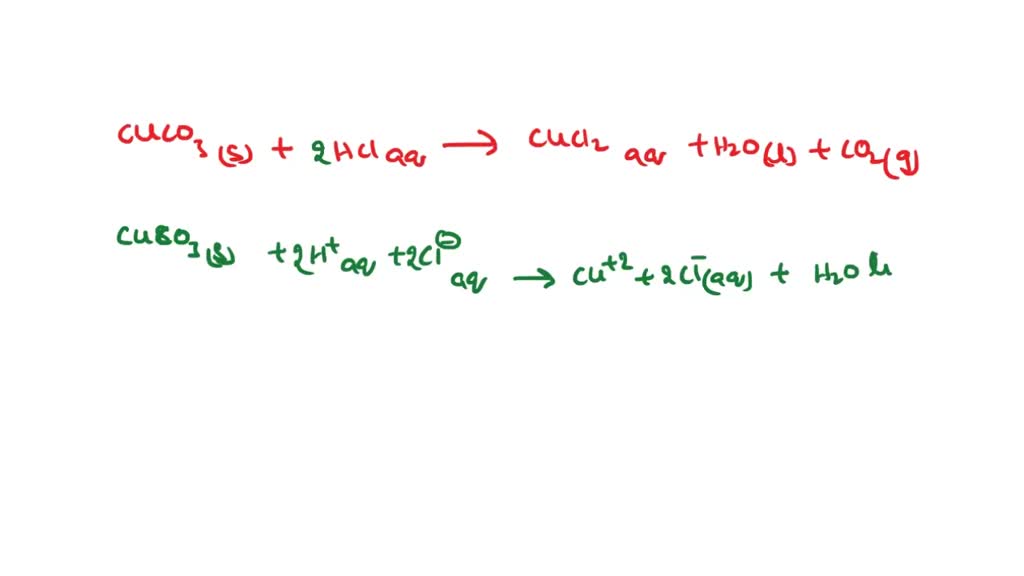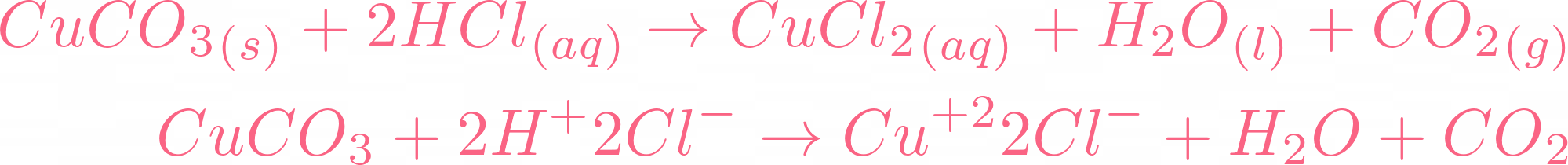Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa
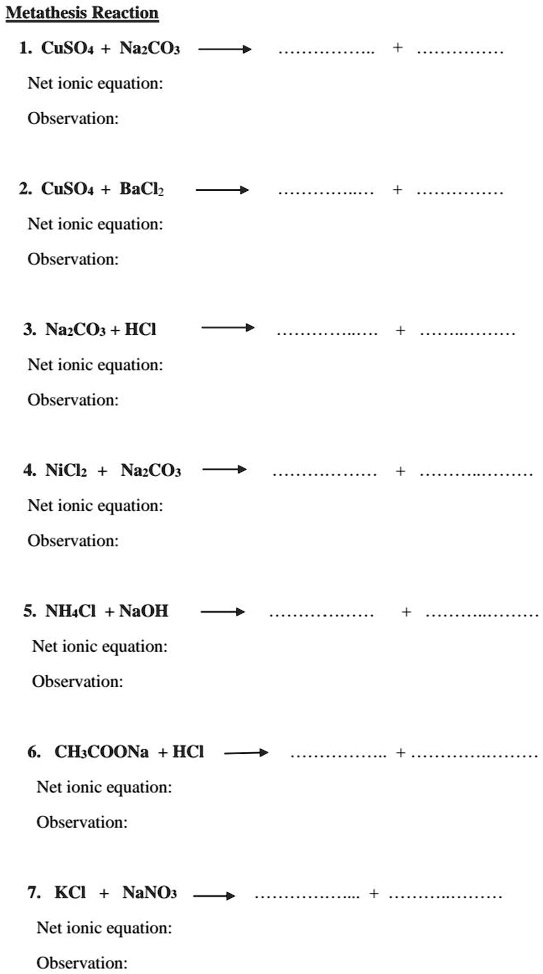
SOLVED: Metathesis Reaction CuSO4 + NaCO3 Net ionic equation: Cu2+ + CO32- â†' CuCO3 Observation: A white precipitate of CuCO3 is formed. 2. Correctedtext: Metathesis Reaction CuSO4 + BaCl2 Net ionic equation:

Example: CuCO3(s) + H2SO4(aq) CuSO4(aq) CO2(g) + H2O Add the excess solid to the acid until no more reacts. Heating may be required Filter off the excess. - ppt download